Emergency use of the anti-malaria drug hydroxychloroquine as a treatment for coronavirus has been withdrawn by the US Food and Drug Administration.
The US Food and Drug Administration said that new evidence from clinical trials meant that it was no longer reasonable to believe that the drug would produce an antiviral effect.
US President Donald Trump later defended promoting the use of hydroxychloroquine as a treatment of Covid-19.
In March, the US Food and Drug Administration granted the emergency use of the drug for some serious cases.
But on Monday, the agency said clinical studies had suggested that the drug was ineffective in treating the deadly virus and failed to prevent infection among those exposed to it. Responding to the US Food and Drug Administration's decision, Trump said he had previously taken the drug preventatively with no side effects.
The 74-year-old president said that many people had told him it had saved their lives.
In May, Trump revealed that he was taking the drug after some people in the White House tested positive for coronavirus.
[Source: BBC]
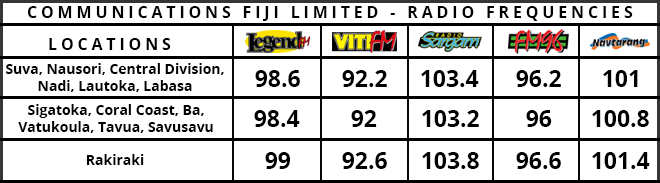
Stay tuned for the latest news on our radio stations