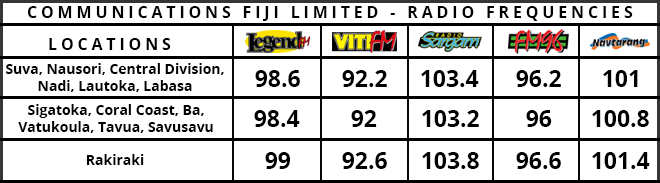
US biotech firm Moderna has shipped an experimental Coronavirus vaccine to US government researchers just six weeks after it started working on the immunisation.
Initial trials of the potential vaccine could begin in April, but the process of testing and approvals would last at least a year.
Moderna added, the first vials of the experimental vaccine would be used in a planned Phase 1 study in the United States, which typically involves testing a vaccine on a small number of healthy humans.
National Institute of Allergy and Infectious Diseases Director Anthony Fauci said that a clinical trial could start by the end of April, the "first step" in potentially making a vaccine available for use.
Fauci told CNN that 45 people would participate in the trial.
Even if the clinical trial is successful, further testing and regulatory approvals would be needed before the vaccine could be deployed widely.
Fauci previously told CNN that researchers could expedite the approval process for a vaccine following a successful Phase 1 trial in an attempt to halt the spread of the virus. But even when proceeding at an "emergency speed," a vaccine would not be available for use for at least a year or 18 months.
Moderna is not the only drug company hoping to find an immunisation for the virus.
Pharma giants Johnson & Johnson (JNJ) and GlaxoSmithKline (GLAXF) are working on vaccines, as are government scientists including some at NIAID.
The novel coronavirus has killed more than 2,800 people worldwide, the vast majority in mainland China. There have been more than 83,000 global cases, with infections in every continent except Antarctica.
[Source:CNN]
Stay tuned for the latest news on our radio stations