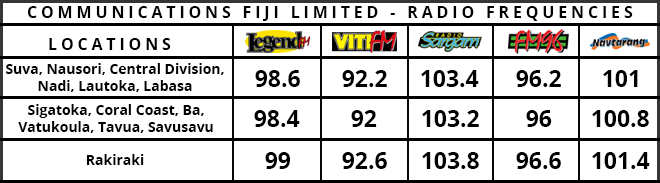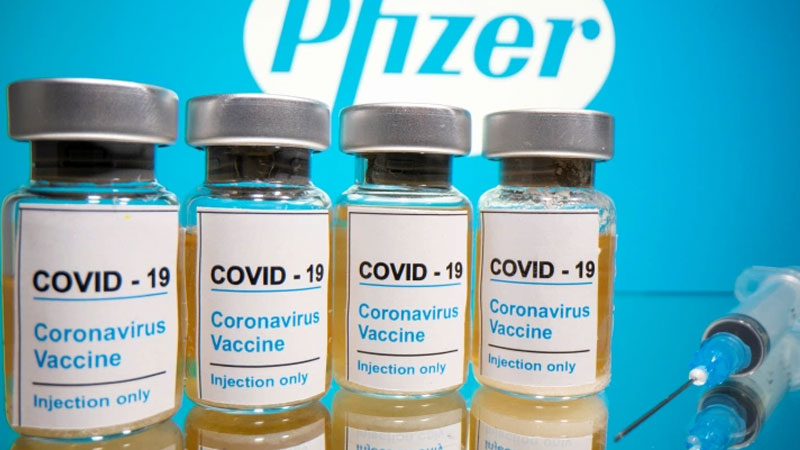
American multinational pharmaceutical corporation, Pfizer and its partner BioNTech have filed for emergency authorisation in the US for their COVID-19 vaccine.
It will be the job of the US Food and Drug Administration to decide if the vaccine is safe to roll out.
BBC News reports it is not clear how long the FDA will take to study the data.
However, the US government expects to approve the vaccine in the first half of December.
BBC News reports data from an advanced trial showed the vaccine protects 94% of adults over 65.
The trial involved 41,000 people worldwide.
Half were given the vaccine, and half a placebo which is is a substance or treatment which is designed to have no therapeutic value.
There are now more than 56 million global COVID-19 cases with 1.3 million deaths according to data collated by Johns Hopkins University in the US.
[Source: BBC]
Stay tuned for the latest news on our radio stations