BioNTech and Pfizer say a three-shot course of their COVID-19 vaccine is able to neutralise the new Omicron variant in a laboratory test, an early signal that booster shots could be key to protection against infection from the newly identified variant.
The German and U.S. companies say two doses of their vaccine resulted in significantly lower neutralising antibodies but could still be protective against severe disease.
Reuters reports that BioNTech Chief Medical Officer Ozlem Tuereci said at a press conference that the first line of defence, with two doses of vaccination, might be compromised and three doses of vaccination are required to restore protection.
The companies also said they could deliver an upgraded vaccine targeted specifically at the Omicron variant in March 2022 if one is needed.
BioNTech and Pfizer are the first manufacturers of a COVID vaccine to issue an official update on the efficacy of their shot against Omicron.
In samples of blood taken around a month after the third shot the Omicron variant was neutralised about as effectively as two doses neutralised the original virus identified in China.
The Omicron variant, first detected in southern Africa and Hong Kong last month, has triggered global alarm about another surge in infections. Cases have already been reported from Japan to the United States and across Europe.
BioNTech CEO Ugur Sahin suggested that countries might consider shortening the time period between second and third doses of the vaccine to combat the new variant.
He cited recent moves by countries including Britain to bring the third shot forward to three months after the second shot, from six months previously.
The World Health Organization classified Omicron on Nov. 26 as a "variant of concern" but said there was no evidence to support the need for new vaccines specifically designed to tackle the variant and its mutations.
Nevertheless, the companies said they would continue efforts to bring an Omicron-specific COVID-19 vaccine to market. Work started on November 25th.
They said their planned production of 4 billion doses of the Comirnaty vaccine in 2022 was not expected to change if an adapted vaccine was required. BioNTech said that even if an adapted vaccine was available in March, it would not be broadly available for some time, noting that perhaps 25 to 75 million doses of the new vaccine would be ready at first.
Pfizer scientist Kena Swanson said the company is considering also testing two doses of an Omicron-specific vaccine in currently unvaccinated people.
There is no significant data yet on how vaccines from Moderna (MRNA.O), Johnson & Johnson (JNJ.N) and other drugmakers hold up against the new variant but they are expected to release their own data within weeks.
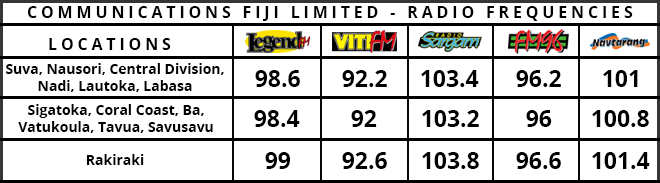
Stay tuned for the latest news on our radio stations