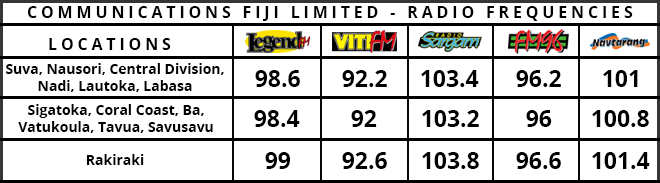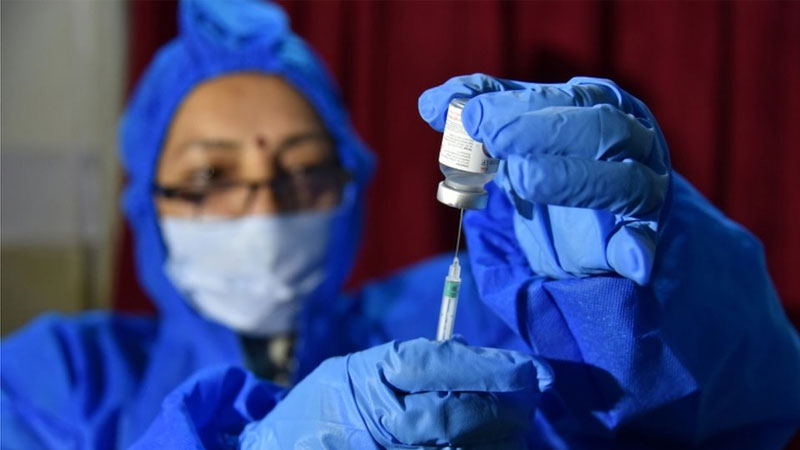
Experts have raised concerns over India's emergency approval of a locally-produced coronavirus vaccine before the completion of trials.
Delhi approved the vaccine - known as Covaxin - as well as the global AstraZeneca Oxford vaccine which is also being manufactured in India.
The head of Bharat Biotech, which makes Covaxin, defended the approval process, but health experts warn it was rushed.
Health watchdog All India Drug Action Network said it was shocking.
It said that there were "intense concerns arising from the absence of the efficacy data" as well a lack of transparency that would "raise more questions than answers and likely will not reinforce faith in scientific decision making bodies".
The statement came after India's Drugs Controller General, VG Somani, insisted Covaxin was "safe and provides a robust immune response".
He added the vaccines had been approved for restricted use in "public interest as an abundant precaution, in clinical trial mode, to have more options for vaccinations, especially in case of infection by mutant strains".
[Source: BBC]
Stay tuned for the latest news on our radio stations