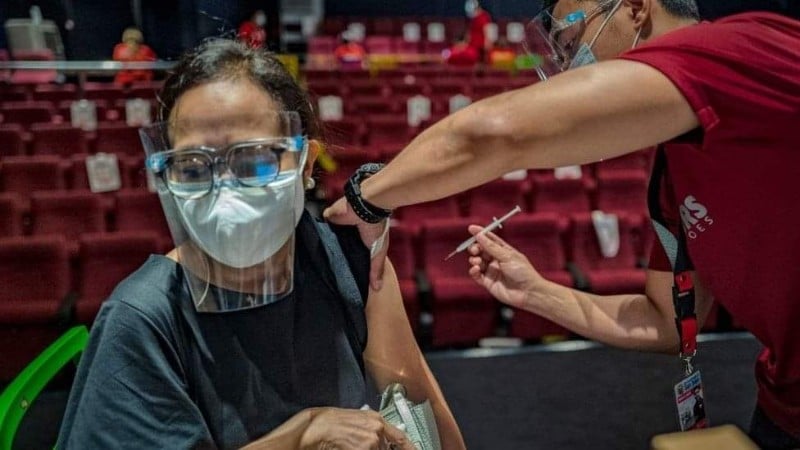
The World Health Organisation has approved China's Sinovac COVID-19 vaccine for emergency use.
It is the second Chinese vaccine to receive the green light from the WHO, after Sinopharm.
It opens the door for the jab to be used in the Covax programme, which aims to ensure fair access to vaccines.
BBC News reports the vaccine, which has already been used in several countries, has been recommended for over 18s, with a second dose two to four weeks later.
The WHO said the emergency approval means the vaccine "meets international standards for safety, efficacy and manufacturing".
According to the WHO, studies showed that Sinovac prevented symptomatic disease in more than half of those vaccinated and prevented severe symptoms and hospitalisation in 100% of those studied.
It is hoped that the decision to list the Chinese vaccine for emergency use will give a boost to the Covax initiative, which has been struggling with supply problems.
One of Sinovac's main advantages is that it can be stored in a standard refrigerator at 2-8 degrees Celsius.
This means Sinovac is a lot more useful to developing countries that might not be able to store large amounts of vaccine at low temperatures.
[Source: BBC]
Stay tuned for the latest news on our radio stations